 [A guest post written primarily by my PhD student Jonathan Lefcheck]
[A guest post written primarily by my PhD student Jonathan Lefcheck]
Have you ever dreamed of living forever? Since the dawn of time people have sought in vain for the fountain of youth. The latest clue to its whereabouts comes from an unlikely source – our closest relative among the invertebrates, the sea squirt. A new study from the University of Gothenberg in Sweden suggests that these humble creatures may carry the secret to eternal life.
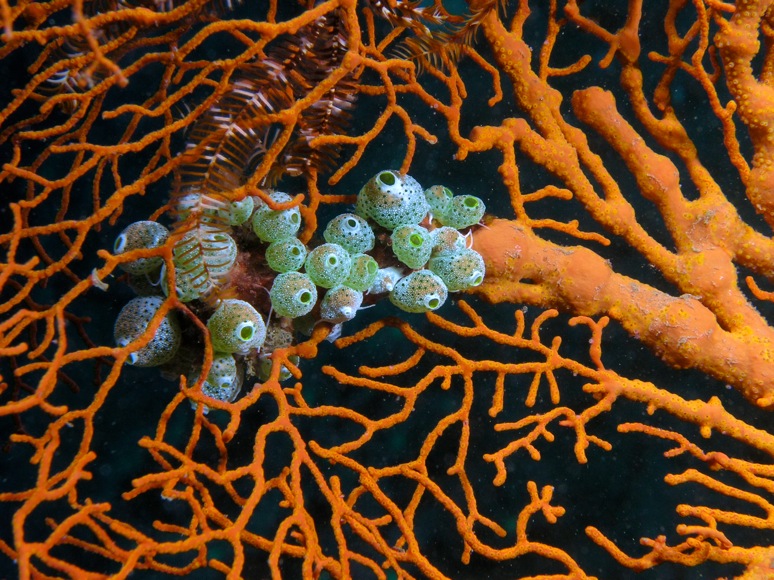
Sea squirts, or ascidians as they’re known to the cogniscenti, are sac-like filter-feeders. They spend their adult lives glued to any hard surface, reaping tiny bits of food from the surrounding water, which they pump continuously through the filter of their bodies. While some sea squirts prefer to go it alone, others connect their tiny bodies together into groups spanning many meters, often in exquisitely beautiful colors and patterns.
Ascidian colonies share a common circulatory system between all of the component individuals, sometimes going so far as to fuse with other, closely-related colonies. It is this ability to distinguish ‘self’ from ‘non-self’ that brought sea squirts to the attention of scientists and helped pioneer early studies of vertebrate immunology.
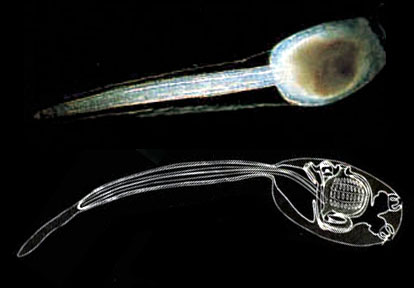
Ascidians are unique among invertebrates (that is, animals without backbones, which means most animals) in that they possess a primitive spine-like organ called a notochord during their early life stages. During this phase the ascidian larva looks remarkably like a tadpole, which is why it is called, you guessed it – a tadpole larva. The flexible, rod-like notochord fingers the sea squirts as chordates, the exalted group that includes humans and all other vertebrates, and in turn separates them from all other invertebrate phyla. While humans possess a notochord as well, it’s only present for a short while. During embryonic development, your notochord is replaced by spinal vertebrae, which help to better protect the delicate spinal cord with hard layers of bone. In fact, the development of the notochord is remarkably similar across chordates, which is why sea squirts have been used as ‘lab rats’ of sorts by developmental biologists. Understanding the development of the nervous system in the relatively simple sea squirts has made major strides in helping us better understand our own, more complex neurological development.
Colonial sea squirts are considered by some to be ‘immortal’ because they reproduce clonally: offspring bud off of the parents as genetically identical copies. If humans could do the same, we could pass on our genetic makeup, if not our personalities (perhaps thankfully), indefinitely. But you as an individual cannot, alas, live forever. Even if you were lucky enough to dodge the many slings and arrows of outrageous fortune during your life, your body is simply not built to last. One of the clearest signs of this inevitable aging is at the cellular level — the degradation of the tips of DNA-containing chromosomes known as telomeres. Because of the way a cell divides and propagates, a little bit of the chromosome is lost every time. By placing a cap of meaningless “gibberish” DNA (i.e., that does not serve the usual purpose of providing instructions) at the end, the cell can avoid deletion of any important information during cell division. Up to a point, that is. Nothing good lasts forever, and problems arise when the telomeres eventually become totally chewed up, because then the coding DNA (that which provides important instructions to the cell’s machinery) is lost and the cells lose function. In other words, they senesce, or age., You might say that the cells reach their built-in expiration date.
 The critical breakthrough discovered by the lowly sea squirt – the secret of eternal life, if you will — is that new clones can regenerate their telomeres, extending the cell’s lifespan by pumping up the amount of DNA that can be chewed off with each cell division. This trick is achieved by an enzyme called telomerase, which is able to do its duty unimpeded as long as the clones are budding. In adults, however, telomerase activity decreases to the point where telomere degradation outpaces replacement. The adult sea squirts then meet the same fate as we do. Alas.
The critical breakthrough discovered by the lowly sea squirt – the secret of eternal life, if you will — is that new clones can regenerate their telomeres, extending the cell’s lifespan by pumping up the amount of DNA that can be chewed off with each cell division. This trick is achieved by an enzyme called telomerase, which is able to do its duty unimpeded as long as the clones are budding. In adults, however, telomerase activity decreases to the point where telomere degradation outpaces replacement. The adult sea squirts then meet the same fate as we do. Alas.
So have we, after so many millennia, finally found the trail to the fountain of youth? Researchers are optimistic that understanding the sea squirt model of telomerase activation offer great promise for improving our understanding of how the same enzyme functions in humans. Perhaps artificially enhancing telomerase activity may one day bestow some of the same rejuvenating properties enjoyed by our lowly cousins (a promising experiment has recently been concluded in mice). Until then, we’re stuck with Oil of Olay.
Original source: Helen Nilsson Sköld and Matthias Obst. 2011. Potential for clonal animals in longevity and ageing studies. Biogerontology DOI: 10.1007/s10522-011-9333-8
Leave a Reply