Hardly a week goes by without a new study being published that documents another negative impact of ocean acidification on ocean life. Now a new paper by Chris Doropoulos (Doropoulos et al 2012) from Ove Hoegh-Guldberg’s lab at UQ reports findings of an experiment suggesting that acidification can even reduce coral recruitment. Crazy!
Coral “recruitment” is the term scientists use to describe the arrival and growth of baby corals. Essentially, this is the stage at which they begin to join the population of juvenile corals attached to the reef after spending weeks or months drifting around in the plankon as traveling vagabonds (technically, the life stage they worked on was settlement, but close enough). When larval (baby) corals settle, they prefer to splash down onto Crustose Corallite Algae (CCA), a type of hard plant that looks like pink cement that corals seem to love.
Recruitment is an essential process for any population. Without it, deaths (and emigration) are not balanced by births and the population eventually goes extinct. This is especially true for threatened organisms like tropical corals, that are having a tough time keeping up with insults including sediment pollution, disease and ocean warming.
Unfortunately, the paper is behind a Blackwell paywall, but here is the abstract:
Successful recruitment in shallow reef ecosystems often involves specific cues that connect planktonic invertebrate larvae with particular crustose coralline algae (CCA) during settlement. While ocean acidification (OA) can reduce larval settlement and the abundance of CCA, the impact of OA on the interactions between planktonic larvae and their preferred settlement substrate are unknown. Here, we demonstrate that CO2 concentrations (800 and 1300 latm) predicted to occur by the end of this century significantly reduce coral (Acropora millepora) settlement and CCA cover by ‡ 45%. The CCA important for inducing coral settlement (Titanoderma spp., Hydrolithon spp.) were the most deleteriously affected by OA. Surprisingly, the only preferred settlement substrate (Titanoderma) in the experimental controls was avoided by coral larvae as pCO2 increased, and other substrata selected. Our results suggest OA may reduce coral population recovery by reducing coral settlement rates, disrupting larval settlement behaviour, and reducing the availability of the most desirable coralline algal species for successful coral recruitment.
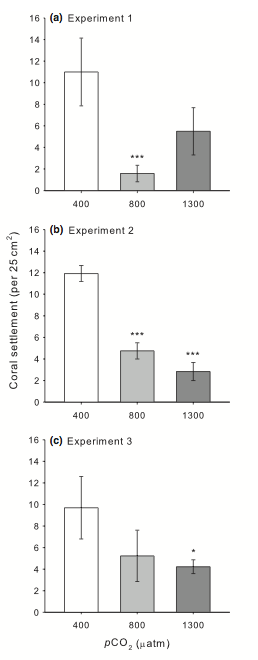 As part of his PhD dissertation, Chris ran three experiments in the experimental OA system at Heron Island Research Station. One in which CCA was exposed to three OA treatments before the coral larve were allowed to settle on it, a second in which the coral larvae were exposed while they were settling, and a third in which both corals and the CCA they settled on were exposed. In all three cases, coral settlement was substantially reduced.
As part of his PhD dissertation, Chris ran three experiments in the experimental OA system at Heron Island Research Station. One in which CCA was exposed to three OA treatments before the coral larve were allowed to settle on it, a second in which the coral larvae were exposed while they were settling, and a third in which both corals and the CCA they settled on were exposed. In all three cases, coral settlement was substantially reduced.
In the authors words: We used a mechanistic approach with three complementary experiments to investigate how OA reduces larval settlement. First, to investigate whether OA caused a shift in the community structure of the settlement substrata to alter coral settlement, we preconditioned settlement tiles in treatment seawater for 60 days prior to conducting 6 day settlement assays on those tiles in ambient seawater (expt. 1). Second, we conducted the reciprocal experiment by isolating the exposure of elevated pCO2 seawater to the coral larvae and settlement substrata during the 6 days settlement phase only (expt. 2). Finally, we explored whether there was a combined effect on coral settlement when the settlement substrata and coral larvae were both exposed to elevated pCO2 for 60 and 6 days respectively (expt. 3). From this series of experiments, we show that OA decreases coral settlement rates by reducing the availability of specific CCA preferred for larval settlement, as well as interfering with the interaction between larvae and CCA by altering the settlement behaviour of the coral larvae, such that previously avoided substrata are preferentially selected as pCO2 increases in all three conditions.
The 400 pCO2 treatments essentially correspond to the current concentration of atmospheric CO2 (which can be seen here). The two higher pCO2 treatments (which will lower ocean pH), 800 and 1300, are values we will reach over the next century or so if we fail to curb carbon emissions. The study indicates that there are two important mechanisms though which acidification could affect coral settlement and recruitment rates; by directly affecting the coral larvae as they settle and begin to calcify their skeleton and by affecting the CCA that they settle on.
I really like several aspects of this experiment. First, the design is pretty elegant (by looking at both potential mechanistic pathways). Second, they pulled off an incredibly difficult feat: performing a serious OA experiment on a remote coral atoll. This type of science is incredibly technical and is a real pain (which is why few other groups are attempting it). Third, it is really nice to see a coral OA experiment that goes beyond looking simply at calcification rates. In general, settlement is a much more important demographic parameter than vertical accretion.
What does it all mean? Well it is just one more of a large and growing body of evidence that OA truly is the other evil twin of climate change. It’s impacts are at times exaggerated and not all the OA science out there is as solid as this work. And the good news is that the really severe effects may not begin for several decades. But the more we learn, the more clear it becomes that we just don’t want to go there. A 1000+ pCO2 word would be a much warmer, more acidic and generally grim place to be a coral. Or a human.
What is ocean acidification? When carbon dioxide (CO2) is absorbed by seawater, chemical reactions occur that reduce seawater pH, carbonate ion concentration, and saturation states of biologically important calcium carbonate minerals. These chemical reactions are termed “ocean acidification” or “OA” for short. Calcium carbonate minerals are the building blocks for the skeletons and shells of many marine organisms. In areas where most life now congregates in the ocean, the seawater is supersaturated with respect to calcium carbonate minerals. This means there are abundant building blocks for calcifying organisms to build their skeletons and shells. However, continued ocean acidification is causing many parts of the ocean to become undersaturated with these minerals, which is likely to affect the ability of some organisms to produce and maintain their shells.
Since the beginning of the Industrial Revolution, the pH of surface ocean waters has fallen by 0.1 pH units. Since the pH scale, like the Richter scale, is logarithmic, this change represents approximately a 30 percent increase in acidity. Future predictions indicate that the oceans will continue to absorb carbon dioxide and become even more acidic. Estimates of future carbon dioxide levels, based on business as usual emission scenarios, indicate that by the end of this century the surface waters of the ocean could be nearly 150 percent more acidic, resulting in a pH that the oceans haven’t experienced for more than 20 million years.
Leave a Reply