This is the sixth installment of my serialization of a new book chapter on “Climate Change and Marine Communities” written with Chris Harley and Mike Burrows. It is for a new book “Marine Community Ecology and Conservation” that I’m co-editing with Mark Bertness, Brian Silliman, and Jay Stachowicz. The book is more or less a followup to the best-selling 2001 edition (which is out of print and worth $100 used and $500 new at Amazon!). We asked our authors to tell us what has happened over the last 10 years in their assigned subfield. The chapters are amazing. And I am truly blown away by how much we’ve discovered since the publication of the first edition! Many fields have been revolutionized and many-a-paradigm has been overturned. Cool stuff.
For more on the basics of Ocean Acidification (OA) go here or download the awesome OA booklet (Mackie et al 2012) here.
Individual- and population-level effects of ocean acidification
Over the last ten years there has been a rapidly growing appreciation and understanding of the threat of OA to marine communities. The OA literature now includes hundreds of experiments measuring effects of OA on a wide range of marine taxa and variables. A meta-analysis (Fig. 8) concluded that OA had “a significant negative effect on survival, calcification, growth, development and abundance. Overall, survival and calcification are the responses most affected by acidification, with 27% reductions in both responses, while growth and development are reduced by approximately 11 to 19%, respectively, for conditions roughly representing year 2100 scenarios” (Kroeker et al. 2013). Additionally, although OA negatively affects many taxa, some such as certain sea stars (Gooding et al. 2009) and some coccolithophores (Iglesias-Rodriguez et al. 2008) benefit from it. These finding indicate that the impact of OA on organisms, populations, and communities could be large, although not uniform and catastrophic as once assumed.
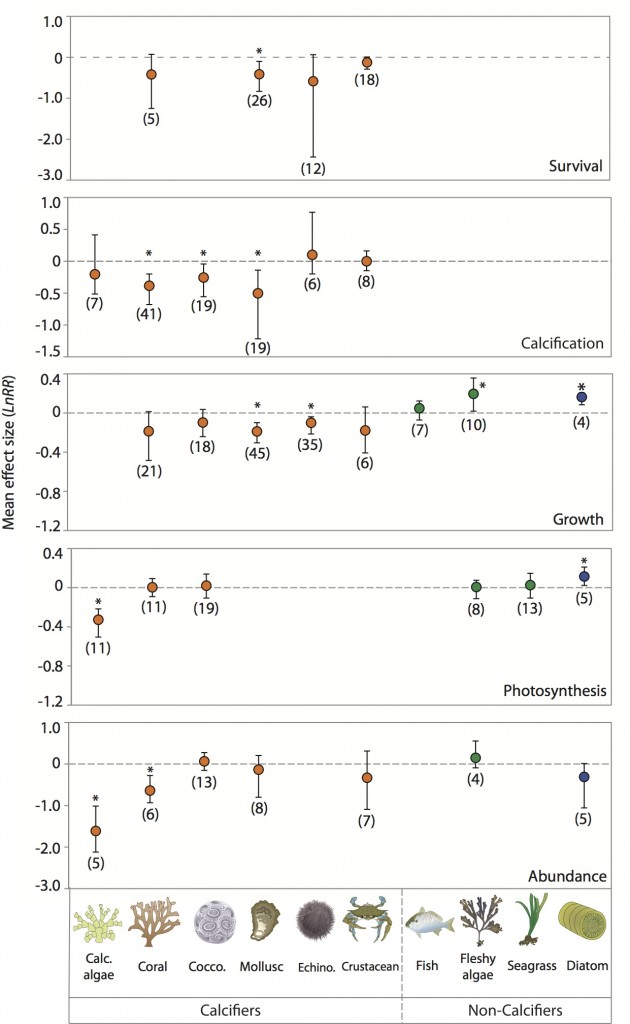 Figure 8. Effect sizes of experimental reduction of pH by 0.5 units from a meta-analysis (Kroeker et al. 2013) for different taxa and responses. Plotted values are means are from a weighted, random-effects model with bootstrapped bias-corrected 95% confidence intervals. The number of experiments used to calculate the means is given in parentheses. * denotes a significant difference from zero.
Figure 8. Effect sizes of experimental reduction of pH by 0.5 units from a meta-analysis (Kroeker et al. 2013) for different taxa and responses. Plotted values are means are from a weighted, random-effects model with bootstrapped bias-corrected 95% confidence intervals. The number of experiments used to calculate the means is given in parentheses. * denotes a significant difference from zero.
The well-developed mechanistic understanding of how OA affects calcification and the ecological implications of losing calcifying foundation species like oysters and corals has focused attention on this process of the many that OA could influence. The reduction in carbonate availability caused by OA has the to potential to directly affect organisms that use calcium carbonate to build their shell or skeleton. Indeed, in numerous laboratory experiments, carbonate ion concentration has been shown to affect rates of calcification and/or skeletal growth in taxa including single-celled crustose coralline algae, crustaceans, corals, molluscs, and echinoderms (Kroeker et al. 2013).
One striking feature of this literature is a high degree of variability in responses to reduced pH among species, higher order taxonomic groupings, life stages, and even among individuals from the same population. Ries et al. (2009) quantified a variety of functional responses to experimental OA, e.g., positive, negative, linear, hump-shaped, etc., based presumably on how well different organisms are able to protect their shells and regulate local pH at the calcification surface (Ries et al. 2009). Scleractinian corals, for example, appear to have substantial pH buffering capacity that enables them to maintain calcification despite reduced external reductions (i.e., in ambient seawater) of carbonate ion concentration (McCulloch et al. 2012). This ability to up-regulate pH at the calcification site by as much as 0.6 pH units, varies among species and does not appear to be present in all taxa (McCulloch et al. 2012).
It has also been shown that corals can use bicarbonate ions (the concentration of which increases with OA) for calcification when the concentration of carbonate ions is reduced (Comeau et al. 2012). Differential ability to employ these and other adaptive mechanisms for coping with changes in pH likely explain both the smaller than expected effects of OA on coral calcification as well as observed variance in responses to experimental OA among coral species (Edmunds et al. 2012). In fact, experimental OA has no measurable effect on some corals such as Pacific massive Porities species (Edmunds et al. 2012) yet substantially reduces calcification of others such as Porities rus (Edmunds et al. 2012). Such differential responses, even within genera, to OA, temperature, and other stressors emphasizes that climate change impacts are more about the changing fortunes of winners and losers (Loya et al. 2001) and altered species composition and community function, than outright community disappearance (see more on altered composition below). It is important to note that although such variability is surprising, it probably shouldn’t have been and it is certainly not unique: organismal and population responses to many other climate change-related factors vary, probably as much as responses to OA.
A meta-analysis of the response of scleractinian corals to experimental acidification (Chan and Connelly 2013) concluded that “under business as usual conditions, declines in coral calcification by end-of-century will be ~22%.” This outcome is similar to the meta-analysis by Kroeker et al. 2013, but contrasts with Edmunds et al (2012), who found little evidence of a general relationship between seawater pH and coral calcification. Such variable outcomes and interpretations unfortunately characterize even the synthetic literature on OA experiments (e.g., see Hendriks et al. 2010 versus Kroeker 2010), obscuring simple take home lessons relevant to policy makers and non-specialists.
The experimental OA literature is also equivocal about whether OA could act additively or synergistically with other stressors, particularly rising temperature. Kroeker et al. (2013) found interactions between these two stressors for some taxa, but no general synergistic effect. There is also substantial ambiguity over the relative sensitivity of different life stages; calcification and survival of some early larval stages appear more sensitive to OA in some taxa such as corals and molluscs (Doropoulos et al. 2012, Talmage 2012) but not in others such as echinoderms or crustaceans and not in general (Kroeker et al. 2013). Interestingly, larval settlement and recruitment appears to be influenced by OA through multiple independent ecological mechanisms. For example, Doropoulos et al. (2012) found that OA can influence coral recruitment by reducing the cover of crustose coralline algal species that facilitate settlement and by altering the settlement behavior and substrate choices of coral larvae. Recent work is also examing “carry-over” effects of larval exposure to OA. For instance, negative effects of OA on larvae of the Pacific Olympia oyster Ostrea lurida appear to carry over and affect settled juveniles long after pH has returned to normal levels (Hettinger et al. 2012). This work highlights the potential long-lasting effects of early and short-term exposure to lowered pH.
Regarding the overall state of the field, a crucial caveat is that very few acidification experiments measure effects on reproduction, overall fitness, susceptibility to other stressors (i.e., in multi-factorial designs) or otherwise attempt to put the results into an ecologically meaningful context – thus we could very well be over- or underestimating the potential impacts of ocean acidification. In fact, we know next to nothing about how or whether the documented effects of acidification scale up to population dynamics. In other words, at this point, we have no idea what the population-level significance of a 25% reduction in calcification would be (Hendriks and Duarte 2010).
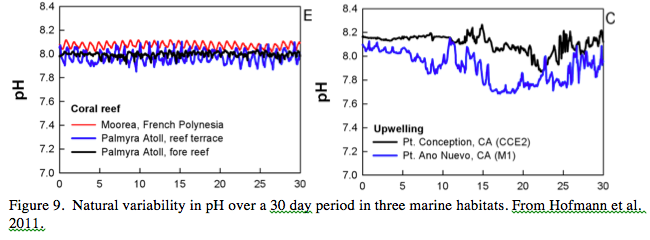
We also do not understand the potential costs of mechanisms that enable calcification in low pH environments, although theoretical work suggests such energetic costs are minimal (McCulloch et al. 2012). And it is important to note that even species that show no response to OA for one response variable, often respond substantially for another. For instance, although OA does not affect the calcification of massive Porities species it does reduce respiration and photochemical efficiency (Edmunds 2012). Finally, the near complete absence of field data on both natural patterns of seawater pH variability and changes due to carbon emissions has greatly hampered the field of OA. New instrumentation (Hofmann et al. 2011) has recently made fine scale and long term field measurements possible and has revealed a huge range of surprisingly variability (Fig. 9). Such variability of pH across space and time could be used to parameterize lab experiments and as treatments in natural field experiments to better understand responses including acclimatization to OA. There is clearly a need to fill in numerous gaps in this rapidly progressing field and developing a better understanding of seawater pH in nature is certainly one of them.
The literature Cited for the entire chapter is here as a PDF
Leave a Reply